
DOFMaster
for Windows
On-line
Depth of Field
Calculator
DOFMaster for Mobile Devices
On-line
Depth of Field
Table
Hyperfocal
Distance Chart
Articles
FAQ
Recommended
Books
Support
Contact
Links
Home
for Windows
On-line
Depth of Field
Calculator
DOFMaster for Mobile Devices
On-line
Depth of Field
Table
Hyperfocal
Distance Chart
Articles
FAQ
Recommended
Books
Support
Contact
Links
Home
As an Amazon Associate I earn from qualifying purchases.
![]()
sometimes stain film. Too little agitation during mixing
may cause the powdered chemicals to settle to the
lumps of chemicals are undissolved and undetected,
the mixer to the storage tank. These lumps can also cause
the solution to be less active.
agitation mixers available. These include large capacity
models for preparing large volumes of solutions and
small models for making small amounts of solution.
foaming and introducing air into the solution. The
solution must be mixed so a minimum amount of air is
drawn into it. When the shaft is placed in the center of
the container, the impeller causes a whirlpool effect that
introduces excessive amounts of air into the solution.
Furthermore, when the shaft is in the center of a
container, there is very little agitation in the bottom-
center area of the container and undissolved chemicals
pile up directly beneath the end of the shaft (fig. 9-5).
the mixer shaft, and a bent shaft produces excessive
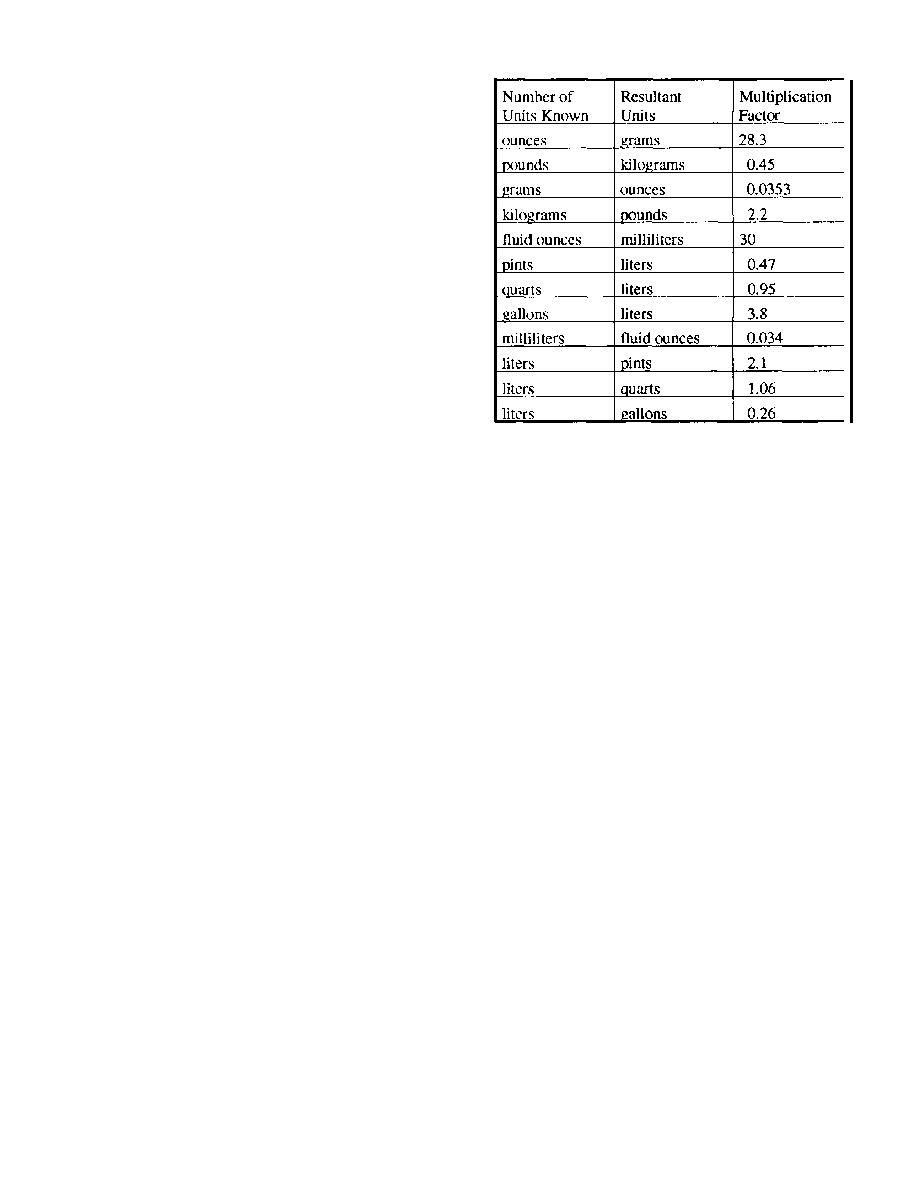
units to one another are matters that every photographer
stand.
weights and measures: avoirdupois and metric. In the
and pounds and are dissolved in pints, quarts, or gallons
fractions or multiples of grams and are dissolved in
cubic centimeters or liters of water. With a conversion
table, a formula given in one system can be easily
converted to the other.
uses °F as a temperature symbol. The Celsius scale
uses °C as its symbol. On the Fahrenheit scale 32
degrees is the freezing point of water, and the boiling
point is 212 degrees. The difference is 180 degrees.
The Celsius scale is 0 to 100 degrees from freezing to
boiling. One degree Fahrenheit is smaller than one
degree Celsius, one Fahrenheit degree being 5/9 of a
Celsius degree. To convert Fahrenheit degrees into
that is, (°F 32) x 5/9 = °C. To convert Celsius to
Fahrenheit, multiply by 9, divide by 5, and add 32; that
is, (°C x 9/5) + 32 = °F.
of another, and any number of parts of water. This is
frequently done when two or more stock solutions must
be combined to make the working solution. In such
cases, the word parts means any convenient "volume"
measurement may be used; however, the same measure
should be used for everything required by the formula.
A part may be a fluid ounce or a gallon, depending upon
the total quantity of working solution needed Formulas
use parts only when volume is to be measured.
used for processing. The working solution may be the
same as the stock solution, but more than likely it is a
Basic Photography Course
As an Amazon Associate I earn from qualifying purchases.
WWW.DOFMASTER.COM
© 2006 Don Fleming. All rights reserved.
© 2006 Don Fleming. All rights reserved.